Patent Issues in CAR-T Technology
While the early hypothesis that chimeric antigen receptors (CARs) could be expressed in T-cells (CAR T-cells) for use as therapeutic agents was more than three decades ago, it was not until 2017 that a CAR-T therapeutic became a reality.
The two products approved by the US Food and Drug Administration (FDA) – Gilead’s YESCARTA and Novartis’s KYMRIAH; which have demonstrated overall response rates of 72% in aggressive B-cell non-Hodgkin lymphoma and 83% in B-cell acute lymphoblastic leukaemia, respectively – have confirmed the therapeutic promise of CAR-T. They are also commercially successful. Yescarta sales in 2019 were $456 million and are predicted to top $1 billion by 2021. Kymriah sales in 2019 were $278 million, and Novartis is projecting that it will reach $1 billion in annual sales within a few years. Indeed, the CAR-T therapy market is expected to be more than $13.5 billion by 2026.
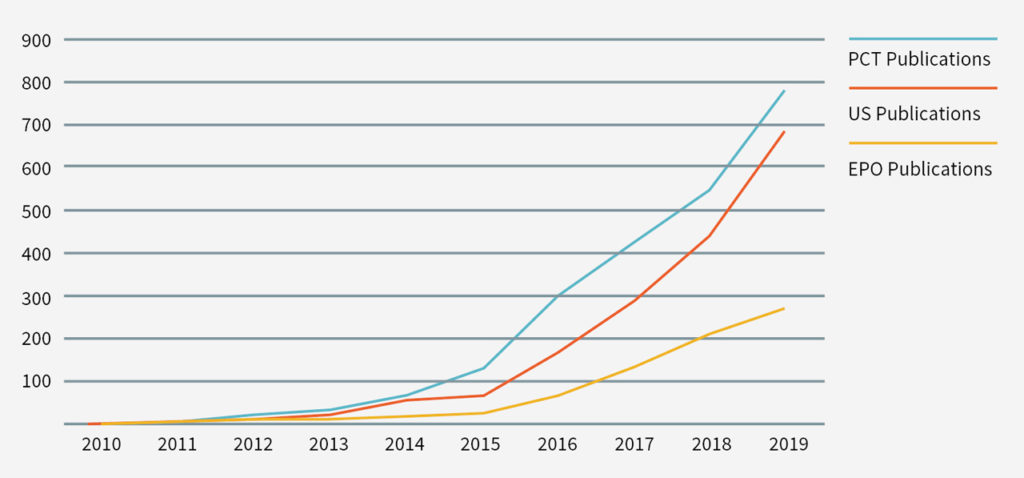
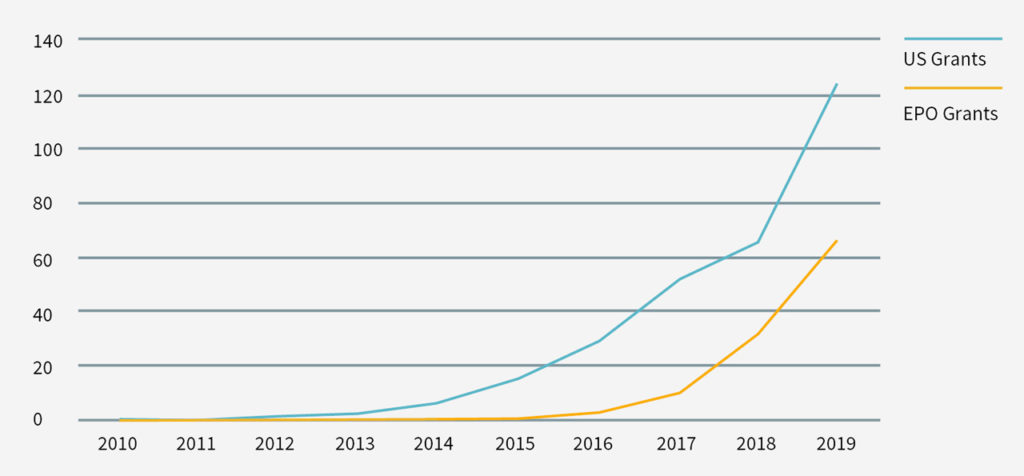
The therapeutic promise of CAR-T technology has also sparked an explosion in research relating to CAR T-cells. By late 2019 there were more than 800 different CAR-T clinical trials ongoing. In addition, the number of patent filings in CAR-T-related technology over the past decade has markedly increased. For example, in the United States, in 2010 there were only three patent publications (including one granted patent) reciting some variation of CAR-T in their title, abstract or claims. By 2019, however, there were 683 CAR-T patent publications (including 124 granted patents) in the United States (see Figures 1 and 2). Similar trends were seen in the Patent Cooperation Treaty and the EPO (see Figures 1 and 2).
A recent patent infringement action in the United States has also demonstrated the value of CAR-T patents. A California jury foundthat Gilead’s Yescarta infringed Juno Therapeutics’ patent directed to a CAR-encoding polynucleotide. The jury awarded Juno $752 million, based, in part, on Gilead’s representation to the US Securities and Exchange Commission (SEC) that Yescarta was worth an estimated $6.2 billion, which the judge enhanced to over $1.1 billion for willful infringement.
In view of the increasingly crowded CAR-T field and the commercial value of CAR-T products and patents, innovators, follow-on developers and late entrants in the CAR-T field should develop early on a sound patent strategy and thoroughly vet their products and processes for any freedom-to-operate issues. Further, any patent strategy relating to the identification of a new disease-associated biomarker or neoantigen should include a CAR-T patent strategy during the early stages of product research and development.
Classification of CAR-T Cells
Patent laws around the world define several categories of invention, including composition of matter, article of manufacture and process. There is some debate within the patent field regarding the category of invention into which CAR-T-cells fall. While this may seem like an esoteric issue, the answer can profoundly affect the ability of a granted patent to protect a CAR-T invention.
On the one hand, CAR-T-cells may be considered physical entities, and as such would be categorised as compositions of matter or articles of manufacture. Under this view, patent applications and patents claiming CAR-T-cells would be evaluated, for both examination and enforcement, in terms of the structural features of the CAR-T-cells and their components. Moreover, because CAR-T-cells express chimeric antigen receptors, which are artificially engineered, they should not be viewed as a product of nature and should accordingly satisfy the threshold eligibility for patent protection in most jurisdictions.
On the other hand, based on the way that they are produced, CAR-T-cells – particularly autogenic CAR-T-cells – may be considered an activity (ie, a therapeutic process or use). The argument supporting this view is that, in the CAR-T process, T-cells are removed from a patient, modified ex vivo to generate CAR-T-cells and administered back to the same patient. As such, these steps define a method of treating each specific patient, not manufacturing a product for more general sale and commercial use. The argument goes that they should not be afforded patent protection as products or compositions of matter.
While methods of treatment are patent eligible subject matter in the United States, many other jurisdictions do not permit the patenting of treatment methods due to concerns of public order and morality. For example, as relevant for CAR-T-cells, the EPO Guidelines for Examination address the ex vivo modification of body tissues and fluids and provide that they “are not excluded from patentability as long as these tissues or fluids are not returned to the same body”. Because autogenic CAR-T-cells are returned to the same body from which they were obtained, their use in a therapeutic method would, at present, seemingly be excluded from patentability. By contrast, allogeneic CAR-T-cells would not be excluded from patentability because the cells are not returned to the same body from which they were obtained. Therefore, characterising CAR-T-cells as a therapeutic process may severely limit the ability to obtain patent protection for them, particularly in many jurisdictions outside the United States. Further, as therapeutic processes, the CAR-T-cells would be evaluated in terms of the actions taken to make or use them, and different actions from those recited in the patent claims could avoid infringement. In order to mitigate these issues, researchers hoping to protect their CAR-T-cell inventions should carefully assess how they characterise them (ie, as products or articles of manufacture or as therapeutic processes). This should be a consistent and global approach and applied to both the researcher’s patent filings and other public disclosures (eg, journal publications, FDA submissions and SEC filings).
Protecting CAR-T inventions – strengths and weaknesses
The strategies for protecting a CAR-T-related invention include protecting:
- the components of the CAR-T-cell;
- the CAR-T-cells themselves;
- methods of manufacturing CAR-T-cells; and
- methods of using CAR-T-cells.
Each strategy has its own strengths and weaknesses. Researchers hoping to protect CAR-T-related inventions should consider a comprehensive and global plan at the outset to pursue each patenting strategy to the fullest extent possible.
In the pharmaceutical and biotech fields, composition of matter claims directed to a particular active ingredient or a formulation comprising an active ingredient are generally the preferred category of claim. Such composition of matter claims should, therefore, be sought in any patent strategy for protecting CAR-T inventions.
For example, patent protection should be sought for the components of a CAR-T-cell, such as the CAR protein expressed in the T-cell and the DNA encoding the CAR protein. The jury verdict in the Yescarta patent infringement litigation demonstrated the value of such patent claims because Juno’s patent was directed to a CAR-encoding polynucleotide. In addition, patent protection should be sought for newly developed functional subunits of a CAR, such as targeting moieties and co-stimulatory domains. For example, it was recently reported that a peptide fragment of scorpion chlorotoxin can be used to effectively target glioblastoma cells. Claims to such peptide fragments and/or the peptide fragments operably linked to the other functional domains of a CAR could broadly protect this targeting mechanism.
Patent protection should also be sought for the CAR-T-cells themselves. To effectively protect CAR-T-cells, care should be taken to characterise them as products or compositions of matter, as opposed to methods of treatment.
Composition of matter claims directed at CAR-T technology provide many of the same advantages that those claim forms offer other pharmaceutical and biotech inventions. For example, such composition of matter claims would protect the CAR-T-cells themselves and their components, independent of how the CAR-T-cells are made and used. Further, to the extent CAR-T-cells are produced outside the United States, US composition-of-matter patents can, in addition to a district court infringement suit, be asserted in the International Trade Commission to obtain an exclusion order preventing import into the United States of the CAR-T products. Once an exclusion order is issued, the burden is on the importer to prove to US Customs that any imported product falls outside of the exclusion order.
CAR-T-related composition of matter patents, however, face certain challenges that composition of matter patents in the pharmaceutical and biotech fields do not. Small molecule drugs and biologics are typically produced for general use and, thus, a patentee can establish infringement for that product or composition generally. Moreover, the product is available for analysis in the context of the elements of the asserted patent claims. By contrast, CAR-T-cells are individually produced for each patient. Therefore, infringement would, in principle, need to be established on a patient-by-patient basis. Further, the CAR-T-cells are infused into the patient shortly after production, so testing to establish that the cells or their components satisfy each of the claim elements may be more challenging or impossible. Accordingly, compared with traditional pharmaceutical and biotech inventions, asserting CAR-T-related composition of matter patents against an infringer may pose some unique challenges. Therefore, method claims should also be a critical component of any patent strategy for CAR-T inventions.
In particular, patent claims directed to methods of manufacturing CAR-T-cells may be particularly valuable. Methods of manufacturing are generally eligible for patent protection in most jurisdictions throughout the world. Indeed, in some jurisdictions, they may be the only option for protecting a medical invention. Further, US patents directed at manufacturing methods can be asserted both in the district court for damages and an injunction, and in the International Trade Commission to obtain an exclusion order to prevent import into the United States of CAR T products produced by the claimed manufacturing methods.
Method of manufacturing claims, however, face challenges that composition of matter claims do not. First, they do not specifically cover the CAR-T product, only the method of making it. Accordingly, to the extent that a competitor can make the same CAR-T product using a different method, it will avoid infringing the claims of the method patent. Further, the manufacturing process for CAR-T-cells involves several different actors, potentially employed and controlled by different entities. For example, the T-cells that are the starting material of a CAR-T product may be obtained by a healthcare professional in a medical facility and then sent to a manufacturing facility owned by another entity for modification. Thus, depending on the steps enumerated in the claims, the owner of a US patent may need to satisfy the heightened standards for divided infringement (ie, that one entity “establishes the manner or timing of [the other’s] performance”). Further, the owner of a US patent may not be able to establish infringement if either the medical facility or the manufacturer is outside the United States. Care should therefore be taken when drafting method of manufacturing claims to use language that is directed to the activities of a single entity.
CAR-T developers should also consider pursuing method of treatment claims. However, such claims are not eligible for patent protection in many jurisdictions outside the United States. While some jurisdictions provide workarounds to the prohibition against method of treatment claims, those workarounds are typically tied to manufacturing methods and use-limited compositions of matter. In addition, method of treatment claims in the United States face the same issues with respect to divided infringement (ie, individual claim elements performed by different actors) and some method steps being performed in the United States and others being performed outside the United States that method of manufacturing claims face. Finally, method of treatment claims necessarily require that an action be taken by a healthcare professional. US patent law, however, limits the extent to which healthcare professionals and their employers are liable for infringing a method of treatment claim. Accordingly, to recover damages for infringement of a method of treatment claim in the United States, a patentee must establish that the CAR-T manufacturer or seller knowingly contributed to or induced infringement. Nevertheless, method of treatment claims are valuable components of any overall patent strategy for protecting CAR-T inventions.
Freedom-to-operate considerations
The CAR-T patent landscape is rapidly growing (see Figures 1 and 2). Accordingly, it is important for participants in the CAR-T field to mitigate the risk of a future infringement suit, or an assertion of wilful infringement and enhanced damages in that action (eg, Yescarta infringement award above) by evaluating their freedom to operate with respect to third-party patents and patents that may issue from pending patent applications.
One important consideration with respect to a freedom-to-operate analysis is the selection of search terms. While a search for patents with terms such as ‘chimeric antigen receptor’, ‘chimeric T-cell receptor’, ‘CAR-T’, ‘Chimeric TCR’, and their variants, will locate a majority of the CAR-T-related patents, it will not identify all CAR-T-related patents. For example, some patents directed at CAR-T inventions use the terms ‘synthetic T-cells’, ‘CAR-modified T-cells’ and ‘recombinant protein’ without ever mentioning ‘chimeric antigen receptor’ or ‘CAR-T’. To increase the chances of identifying these more elusive patents, a comprehensive keyword search should be paired with a structure-based (ie, a sequence-based) search.
By their nature, CAR-T-related inventions also have many different aspects, each of which may be protected by patents independent of the CAR-T context. For example, the targeting moiety (eg, scFV, receptor fragment or toxin fragment) may be patented independently of its use in a CAR-T system. Similarly, various co-stimulatory domains may be separately protected. In addition, CAR-T modifications to limit any toxicity associated with CAR-T therapy are in development and may be separately protected. Efforts are also ongoing to adapt CAR-T therapy for the treatment of solid tumours and to avoid the suppressive effect of a hostile tumour microenvironment. Indeed, some efforts now include modifying macrophages to express a CAR (ie, a CAR-M cell) for use in treating solid tumours. Further, the CAR-encoding polynucleotides are generally integrated into the genome of the patient’s T-cells. In view of their intended therapeutic use, limited vectors (eg, an integrating gammaretrovirus or a lentivirus) and techniques (eg, CRISPR and zinc-finger nucleases) are also available for such integration. These vectors and techniques used for delivering the CAR-encoding polynucleotide may be patented independently of the CAR-T context. Finally, patients receiving CAR-T as a therapeutic may require pre-conditioning (eg, lymphodepletion chemotherapy). Therefore, any comprehensive freedom-to-operate analysis should include an evaluation of patents directed to these various, more general aspects of CAR-T therapy.
To the extent possible, freedom to operate should be evaluated at the early stages of the development of a CAR-T product. Early identification of patents covering aspects of a CAR-T invention permits an innovator to design around the patents, avoiding or reducing a future infringement risk. Alternatively, to the extent that a patent cannot be avoided, early identification provides the innovator with an opportunity to challenge the problematic patent in a post-grant proceeding at a patent office in an attempt to clear the way for its product before launch or an to attempt to license it from the patent owner.
Comment
While only two CAR-T therapies have been approved to date, if the number of clinical trials and patent filings are any indication, they represent the first of many. As companies look to bring new CAR-T (and CAR-M) innovations to market, they should, from the outset, develop a comprehensive strategy for protecting their innovations and minimising the risk of patent infringement in launching their products. They should also take a global approach to ensure that their actions in obtaining and enforcing their patents or challenging a competitor’s patents in one country do not negatively impact on proceedings in other countries. Such a global, comprehensive approach will allow companies to develop and safeguard their competitive advantages, bring their products to market, stimulate future development efforts and improve the health of patients worldwide.
By James F. Haley, Karen Mangasarian, and Brian M. Gummow
Share